The Anesthesia Gas Machine 2026
Michael P. Dosch PhD CRNA (ret), Darin Tharp CRNA MS
University of Detroit Mercy - Nurse Anesthesia
This site is https://healthprofessions.udmercy.edu/academics/na/agm/.
Revised Feb. 2026

The Anesthesia Gas Machine 2026
Michael P. Dosch PhD CRNA (ret), Darin Tharp CRNA MS
University of Detroit Mercy - Nurse Anesthesia
This site is https://healthprofessions.udmercy.edu/academics/na/agm/.
Revised Feb. 2026

Problems:
The strong bases (NaOH, KOH which function as activators) have been convincingly implicated in the carbon monoxide problem with the methyl-ethyl ethers (des-, sevo, isoflurane), and the generation of Compound A by sevoflurane. Two definitive approaches to dealing with these problems have surfaced:
Lithium hydroxide lime (LitholymeTM, Allied Healthcare Products, Inc.) is an effective carbon dioxide absorbent, and is free of the strong bases (NaOH, KOH). Litholyme contains: Ca(OH)2 as the main constiuent by weight and ethyl violet as the indicator (like soda lime), but with LiCl as the catalyst. Litholyme does not contain KOH or NaOH. The CO2 absorbing capacity is similar to Sodasorb and more than Amsorb (see [4] at end). Litholyme does not produce Compound A or carbon monoxide from breakdown of any anesthetic agent under any circumstances even when it is desiccated ((see [4] at end). Data supplied by the manufacturer shows that Litholyme:
Spiralith has lithium hydroxide (rather than calcium hydroxide) as its main constituent. Spiralith is a unique absorbent which is efficient, and does not degrade volatile agents. It does not desiccate since its water content is chemically bound. Spiralith is a powder enclosed in a polymer sheet, making the absorbent recyclable, and also limiting the danger to eyes and skin posed by dust in traditional absorbents. Since it lacks a color indicator, it may only be used with capnography monitoring.
Both Litholyme and Spiralith were found to be quite efficient in Aisys canisters.[5]. Lithium hydroxide is a mature technology with a long history of use in space and submarine environments (though somewhat new in anesthesia applications).
Eliminating the activators entirely produces an absorbent which has similar physical characteristics (but slightly less carbon dioxide absorption efficiency), as compared to soda lime: Amsorb (Armstrong Medical Ltd., Coleraine Northern Ireland).[6], [7]
|
|
CLIC absorber on Apollo. Note Draeger open scavenger interface on left. Click on the link to see the larger version. New "house brand" absorbents have been created to help deal with the problems of modern volatile anesthetic (desflurane, sevoflurane) breakdown. "House brand" absorbents made by GE and Dräger have less activator, especially less KOH. Dräger makes an absorbent with decreased amounts of NaOH, and no KOH (Drägersorb Free) which will not generate Compound A or carbon monoxide under any circumstances (low flows, or desiccated granules.[8] GE makes Medisorb, which also has decreased amounts of strong bases. |
For all:
| Component | Soda lime | Medisorb | Dragersorb Free | Amsorb Plus | Litholyme | Spiralith |
| Ca(OH)2 % | 94 | 70-80 | 74-82 | >80 | >75 | - |
| NaOH % (activator) | 2-4 | 1-2 | 0.5-2 | - | - | - |
| KOH % (activator) | 1-3 | 0.003 | 0 | - | - | - |
| LiOH % | - | - | - | - | - | 95 |
| Li2CO3 % | - | - | - | - | - | 3 |
| Lithium chloride % (catalyst) | - | - | - | - | < 3 | - |
| Water Content % | 14-19 | 16-20 | 14-18** | 13-18** | 12-19 | Unknown* |
| Size (mesh) | 4-8 | 4-8 | 4-8 | 4-8 | 4-8 | -* |
| Indicator | Yes | Yes | Yes | Yes | Yes | No |
* The water in Spiralith is chemically bound, so it does not desiccate. It does not have a granular size, since absorbent powder is encased in a polymer matrix, and it does not have an indicator.
** Draegersorb Free and Amsorb Plus contain CaCl2 as a humectant (helps retain moisture).
Numbers are approximations which may not sum to 100%.
Data sources for this table.[10], [11], [12], [13], [14], [15], [16]
While it is possible to change canisters mid-case, it is safer to wait until the end of the case to do so. There are many case reports of inability to ventilate due to leaks after canisters could not be fit back together.[17], [18], [19], [20]

|
Canister change- Aisys. Click on the link to see the larger version. To change the absorbent granule canister on Aisys, press the latch release (arrow at left). The canister will pivot, and may then be slid off its rails (right). A new canister is then slid on, and pivoted upwards to latch. |

|
CLIC canister change- Apollo. Click on the link to see the larger version. The Draeger CLIC canister (Apollo, Perseus) is changed in a similar way. |
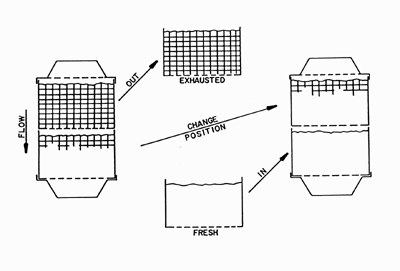
|
Steps for changing dual canister (e.g. Aestiva) canisters. Click on the link to see the larger version.
|
Inspiratory and expiratory unidirectional valves which are functioning properly direct all exhaled gases through the absorbent granules. Provided these granules are intact (non-exhausted), and not channeling or otherwise inactivated, there can be no inspired carbon dioxide (i.e. FICO2 should be zero even if CO2 production is increased e.g. fever, sepsis).
Capnography (and properly set alarms) allow us to change canisters when inspired carbon dioxide exceeds a known threshold (3-5 cm H2O). Using FICO2 as the trigger for absorbent granule change is a more efficient use of granules than relying on color change.[21]
Signs of elevated inspired CO2
[1] Kennedy RR, Hendrickx JF, Feldman JM. There are no dragons: Low-flow anaesthesia with sevoflurane is safe. Anaesth Intensive Care. 2019 May;47(3):223-225. doi: 10.1177/0310057X19843304.
[2] ASA Committee on Equipment and Facilities. Statement on the Use of Low Gas Flows for Sevoflurane (2023) at https://www.asahq.org/standards-and-practice-parameters/statement-on-the-use-of-low-gas-flows-for-sevoflurane Accessed Feb 1, 2026
[3] Allied Healthcare Products Inc. Litholyme Material Safety Data Sheet. At https://assets-mirion.mirion.com/prod-20220822/cms4_mirion/files/capintec/PDF/compliance-certificates/sds-litholyme-alliedhealthcare.pdf Published 2023. Accessed Jan 26, 2026.
[4] Dahms T, Statzer N, Haynes G. Evaluation of a new carbon dioxide absorber: Litholyme. Proceedings of the 2010 Annual Meeting of the American Society of Anesthesiologists at https://www.alliedmedgas.org/Brochures/A/Allied_Healthcare/LithoLyme-2.pdf See p. 3-8. Accessed Feb 6, 2026
[5] Hendrickx JF, De Ridder SP, Dehouwer A, Carette R, De Cooman S, De Wolf AM. In vitro performance of prefilled CO2 absorbers with the Aisys®. J Clin Monit Comput. 2016 Apr;30(2):193-202. doi: 10.1007/s10877-015-9699-2.
[6] Zilberman, Paul. (2015). The CO2 Absorber Based on LiOH. Acta Medica Marisiensis. 61. 10.1515/amma-2015-0023.
[7] Versichelen LF, Bouche MP, Rolly G, Van Bocxlaer JF, Struys MM, De Leenheer AP, Mortier EP. Only carbon dioxide absorbents free of both NaOH and KOH do not generate compound A during in vitro closed-system sevoflurane: evaluation of five absorbents. Anesthesiology. 2001 Sep;95(3):750-5. doi: 10.1097/00000542-200109000-00030.
[8] Struys MM, Bouche MP, Rolly G, Vandevivere YD, Dyzers D, Goeteyn W, Versichelen LF, Van Bocxlaer JF, Mortier EP. Production of compound A and carbon monoxide in circle systems: an in vitro comparison of two carbon dioxide absorbents. Anaesthesia. 2004 Jun;59(6):584-9. doi: 10.1111/j.1365-2044.2004.03704.x.
[9] Pond D, Jaffe RA, Brock-Utne JG. Failure to detect CO2-absorbent exhaustion: seeing and believing. Anesthesiology. 2000 Apr;92(4):1196-8. doi: 10.1097/00000542-200004000-00041.
[10] Olympio MA. Carbon Dioxide Absorbent Desiccation Safety Conference Convened by APSF https://www.apsf.org/article/carbon-dioxide-absorbent-desiccation-safety-conference-convened-by-apsf/ Accessed Feb 1, 2026
[11] Higuchi H, Adachi Y, Arimura S, Kanno M, Satoh T. The carbon dioxide absorption capacity of Amsorb is half that of soda lime. Anesth Analg. 2001 Jul;93(1):221-5. doi: 10.1097/00000539-200107000-00044.
[12] Wissing H, Kuhn I, Warnken U, Dudziak R. Carbon monoxide production from desflurane, enflurane, halothane, isoflurane, and sevoflurane with dry soda lime. Anesthesiology. 2001 Nov;95(5):1205-12. doi: 10.1097/00000542-200111000-00026.
[13] Yamakage M, Yamada S, Chen X, Iwasaki S, Tsujiguchi N, Namiki A. Carbon dioxide absorbents containing potassium hydroxide produce much larger concentrations of compound A from sevoflurane in clinical practice. Anesth Analg. 2000 Jul;91(1):220-4. doi: 10.1097/00000539-200007000-00041.
[14] Micropore. Spiralith absorbent technical brochure. https://spiralithusa.com/product-support/#papers Accessed Feb 1, 2026
[15] Allied Medical LLC. Litholyme MSDS. https://assets-mirion.mirion.com/prod-20220822/cms4_mirion/files/capintec/PDF/compliance-certificates/sds-litholyme-alliedhealthcare.pdf, Accessed Feb 1, 2026
[16] Struys MM, Bouche MP, Rolly G, Vandevivere YD, Dyzers D, Goeteyn W, Versichelen LF, Van Bocxlaer JF, Mortier EP. Production of compound A and carbon monoxide in circle systems: an in vitro comparison of two carbon dioxide absorbents. Anaesthesia. 2004 Jun;59(6):584-9. doi: 10.1111/j.1365-2044.2004.03704.x.
[17] Maruyama K, Akihisa Y, Andoh T. Massive gas leak following misconnection of a Datex-Ohmeda Multi Absorber Disposable canister, Br J Anaesth 2011;107(eLetters Supplement):No Pagination Specified. doi: 10.1093/bja/el_7463
[18] Umesh G, Jasvinder K, Sagarnil R. Leak in the breathing circuit: CO2 absorber and human error. J Clin Monit Comput. 2010 Apr;24(2):143-4. doi: 10.1007/s10877-010-9223-7.
[19] Kummar P, Korula G, Kumar S, Saravanan PA. Unusual cause of leak in Datex Aisys. Anesth Analg. 2009 Oct;109(4):1350-1; discussion 1351-2. doi: 10.1213/ane.0b013e3181b2a931.
[20] Seif DM, Olympio MA. Expiratory limb ventilation during unique failure of the anesthesia machine breathing circuit. Anesthesiology. 2013 Mar;118(3):751-3. doi: 10.1097/ALN.0b013e318282a32c.
[21] Feldman JM, Hendrickx J, Kennedy RR. Carbon Dioxide Absorption During Inhalation Anesthesia: A Modern Practice. Anesth Analg. 2021 Apr 1;132(4):993-1002. doi: 10.1213/ANE.0000000000005137.