The Anesthesia Gas Machine 2026
Michael P. Dosch PhD CRNA (ret), Darin Tharp CRNA MS
University of Detroit Mercy - Nurse Anesthesia
This site is https://healthprofessions.udmercy.edu/academics/na/agm/.
Revised Feb. 2026

The Anesthesia Gas Machine 2026
Michael P. Dosch PhD CRNA (ret), Darin Tharp CRNA MS
University of Detroit Mercy - Nurse Anesthesia
This site is https://healthprofessions.udmercy.edu/academics/na/agm/.
Revised Feb. 2026
One of the things I notice about the practice of anesthesia is the extensive use of protocols and procedures. As I learn more about anesthesia I realize how important protocols and procedures are to increase patient safety.
As a lawyer I also see that these procedures can protect the anesthetist. Should the anesthetist be required to defend himself or herself, it may be difficult to remember the exact details of an anesthetic given years before. Sometimes, it is helpful to be able to testify that certain matters are always done by following careful procedures, even if you cannot remember what happened in a particular case. Giving an anesthetic clearly requires thought and judgment, but the importance of having and following procedures can not be minimized. If you begin your day or each operation checking out your anesthesia machine... then even if you cannot remember what you did on February 1, 1995, you will know you checked the anesthesia machine because that is what you always do.
--Gene Blumenreich AANA Journal 2000;68:107.
Prior to the late 1980s, gas machine operator's manuals contained preoperative checks for each model. These were exhaustive, reflecting an engineer's perspective, not a clinician's.
A checklist usable for all gas machines was proposed by the professions and accepted by the FDA in 1987 (and revised in 1993). The 1993 checklist has been relatively well-studied. It has been found that users did not use the checklist consistently, and that it was not effective in discovering faults. Further, modern gas machines with sophisticated electronic controls have automated checkout routines built in, and thus it is not possible to use the FDA checklist on them.
The 1993 FDA checklist has been superseded by the latest revision. [1] The Pre-Anesthesia Checkout (PAC) 2008 is principles-based (ie what to check), since no one procedural checklist ie how to check it) applies to all modern gas machine models. Therefore, it is envisioned that each department will prepare a procedure list which is appropriate for checkout of each type of gas machine they use.
As recently as 2013 it was reported that failure to check a disposable breathing circuit contributed to a patient fatality. [2] The Closed Claims study of gas delivery equipment concluded that The majority (85%) of claims involved provider error with (n = 7) or without (n = 27) equipment failure. Thirty-five percent of claims [and 75% of breathing circuit claims] were judged as preventable by preanesthesia machine check. [3]
|
Step
|
Rationale |
|---|---|
|
Failure to be able to ventilate is a major cause of morbidity and mortality related to anesthesia care. Because equipment failure with resulting inability to ventilate the patient can occur at any time, a self-inflating manual ventilation device (eg. AMBU bag) should be present at every anesthetizing location for every case and should be checked for proper function. In addition, a source of oxygen separate from the anesthesia machine and pipeline supply, specifically an oxygen cylinder with regulator and a means to open the cylinder valve, should be immediately available and checked. After checking the cylinder pressure, it is recommended that the main cylinder valve be closed to avoid inadvertent emptying of the cylinder through a leaky or open regulator. |
|
Safe anesthetic care requires the immediate availability of suction to clear the airway if needed. |
|
Anesthesia delivery systems typically function with backup battery power if AC power fails. Unless the presence of AC power is confirmed, the first obvious sign of power failure can be a complete system shutdown when the batteries can no longer power the system. Many anesthesia delivery systems have visual indicators of the power source showing the presence of both AC and battery power. These indicators should be checked and connection of the power cord to a functional AC power source should be confirmed. Desflurane vaporizers require electrical power and recommendations for checking power to these vaporizers should also be followed. |
|
Standards for patient monitoring during anesthesia are clearly defined. The ability to conform to these standards should be confirmed for every anesthetic.
|
|
Anesthesia delivery systems rely on a supply of oxygen for various machine functions. At a minimum, the oxygen supply is used to provide oxygen to the patient. Pneumatically-powered ventilators also rely on a gas supply. Oxygen cylinder(s) should be mounted on the anesthesia delivery system and determined to have an acceptable minimum pressure. The acceptable pressure depends on the intended use, the design of the anesthesia delivery system and the availability of piped oxygen. Typically, an oxygen cylinder will be used if the central oxygen supply fails.
The oxygen cylinder valve should be closed after it has been verified that adequate pressure is present, unless the cylinder is to be the primary source of oxygen (i.e. piped oxygen is not available). If the valve remains open and the pipeline supply should fail, the oxygen cylinder can become depleted while the anesthesia provider is unaware of the oxygen supply problem. Other gas supply cylinders (e.g. Heliox, CO2, Air, N2O) need to be checked only if that gas is required to provide anesthetic care. |
|
A minimum gas supply pressure is required for proper function of the anesthesia delivery system. Gas supplied from a central source can fail for a variety of reasons. Therefore the pressure in the piped gas supply should be checked at least once daily. |
|
If anesthetic vapor delivery is planned, an adequate supply is essential to reduce the risk of light anesthesia or recall. This is especially true if an anesthetic agent monitor with a low agent alarm is not being used. Partially open filler ports are a common cause of leaks that may not be detected if the vaporizer control dial is not open when a leak test is performed. This leak source can be minimized by tightly closing filler ports. Newer vaporizer designs have filling systems that automatically close the filler port when filling is completed. High and low anesthetic agent alarms are useful to help prevent over- or under-dosage of anesthetic vapor. Use of these alarms is encouraged and they should be set to the appropriate limits and enabled. |
|
The gas supply in this part of the anesthesia delivery system passes through the anesthetic vaporizer(s) on most anesthesia delivery systems. In order to perform a thorough leak test, each vaporizer must be turned on individually to check for leaks at the vaporizer mount(s) or inside the vaporizer. Furthermore, some machines have a check valve between the flowmeters and the common gas outlet, requiring a negative pressure test to adequately check for leaks. Automated checkout procedures typically include a leak test but may not evaluate leaks at the vaporizer especially if the vaporizer is not turned on during the leak test. When relying upon automated testing to evaluate the system for leaks, the automated leak test would need to be repeated for each vaporizer in place. This test should also be completed whenever a vaporizer is changed. The risk of a leak at the vaporizer depends upon the vaporizer design. Vaporizer designs where the filler port closes automatically after filling can reduce the risk of leaks. |
|
A properly functioning scavenging system prevents room contamination by anesthetic gases.
|
|
Continuous monitoring of the inspired oxygen concentration is the last line of defense against delivering hypoxic gas concentrations to the patient. The oxygen monitor is essential for detecting adulteration of the oxygen supply.
|
|
Proper function of a circle anesthesia system relies on the absorbent to remove carbon dioxide from rebreathed gas. Exhausted absorbent as indicated by the characteristic color change should be replaced. It is possible for absorbent material to lose the ability to absorb CO2 yet the characteristic color change may be absent or difficult to see. Some newer absorbents do change color when desiccated. Capnography should be utilized for every anesthetic and, when using a circle anesthesia system, rebreathing carbon dioxide as indicated by an inspired CO2 concentration > 0 mm Hg can also indicate exhausted absorbent. |
|
The breathing system pressure and leak test should be performed with the circuit configuration to be used during anesthetic delivery. If any components of the circuit are changed after this test is completed, the test should be performed again. Although the anesthesia provider should perform this test before each use, anesthesia technicians who replace and assemble circuits can also perform this check and add redundancy to this important checkout procedure. Proper testing will demonstrate that pressure can be developed in the breathing system during both manual and mechanical ventilation and that pressure can be relieved during manual ventilation by opening the APL valve. Automated testing is often implemented in the newer anesthesia delivery systems to evaluate the system for leaks and also to determine the compliance of the breathing system. The compliance value determined during this testing will be used to automatically adjust the volume delivered by the ventilator to maintain a constant volume delivery to the patient. It is important that the circuit configuration that is to be used be in place during the test. |
|
Pressure and leak testing does not identify all obstructions in the breathing circuit or confirm proper function of the inspiratory and expiratory unidirectional valves. A test lung or second reservoir bag can be used to confirm that flow through the circuit is unimpeded. Complete testing includes both manual and mechanical ventilation. The presence of the unidirectional valves can be assessed visually during the PAC. Proper function of these valves cannot be visually assessed since subtle valve incompetence may not be detected. Checkout procedures to identify valve incompetence which may not be visually obvious can be implemented but are typically too complex for daily testing. A trained technician can perform regular valve competence tests. Capnography should be used during every anesthetic and the presence of carbon dioxide in the inspired gases can help to detect an incompetent valve. |
|
Each individual responsible for checkout procedures should document completion of these procedures. Documentation gives credit for completing the job and can be helpful if an adverse event should occur. Some automated checkout systems maintain an audit trail of completed checkout procedures that are dated and timed. |
|
This step is intended to avoid errors due to production pressure or other sources of haste. The goal is to confirm that appropriate checks have been completed and that essential equipment is indeed available. The concept is analogous to the “time out” used to confirm patient identity and surgical site prior to incision. Improper ventilator settings can be harmful especially if a small patient is following a much larger patient or vice versa. Pressure limit settings (when available) should be used to prevent excessive volume delivery from improper ventilator settings.
|
|
Timing |
Perform the entire checklist daily. Repeat the following items before each case:
|
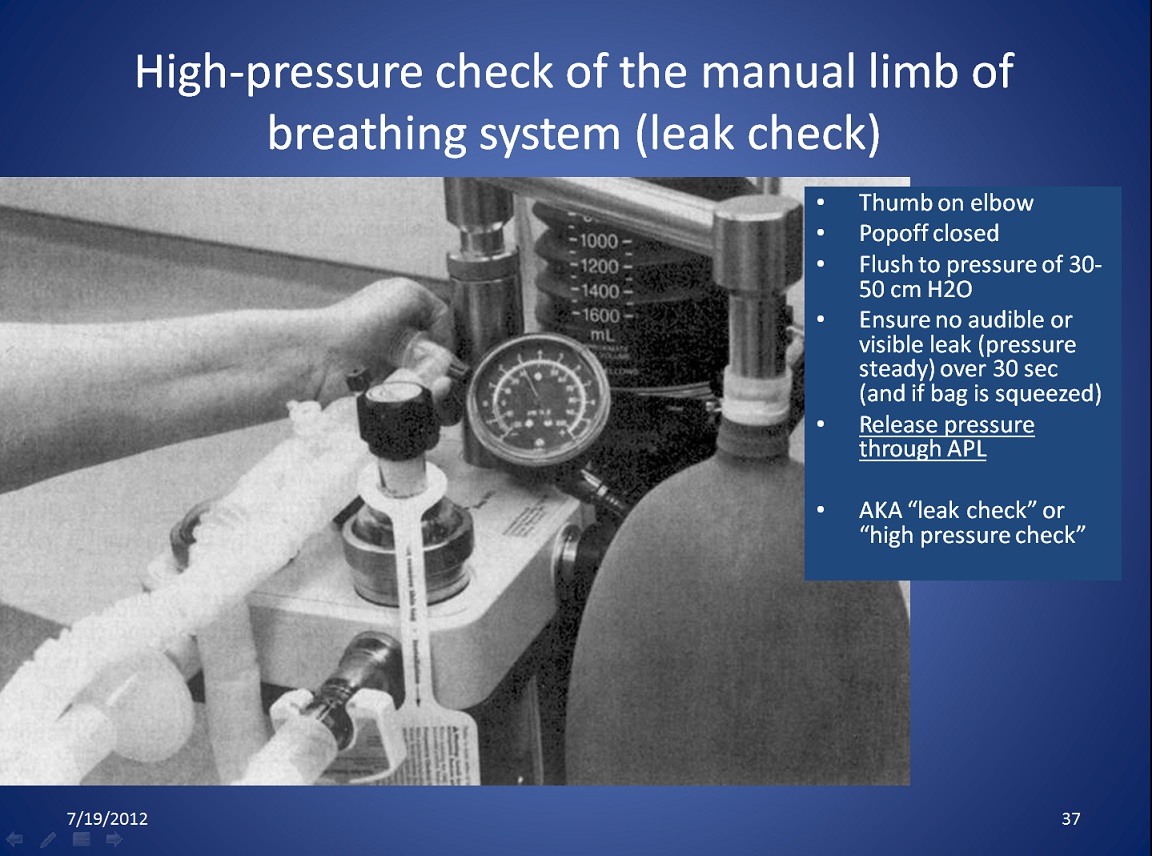
|
How to do a high pressure Leak Check. Click on the link to see the larger version. A superior method of testing for leaks (step 12 above), obstruction and unidirectional valve function (step 13 above) is detailed below- the PaF test. |
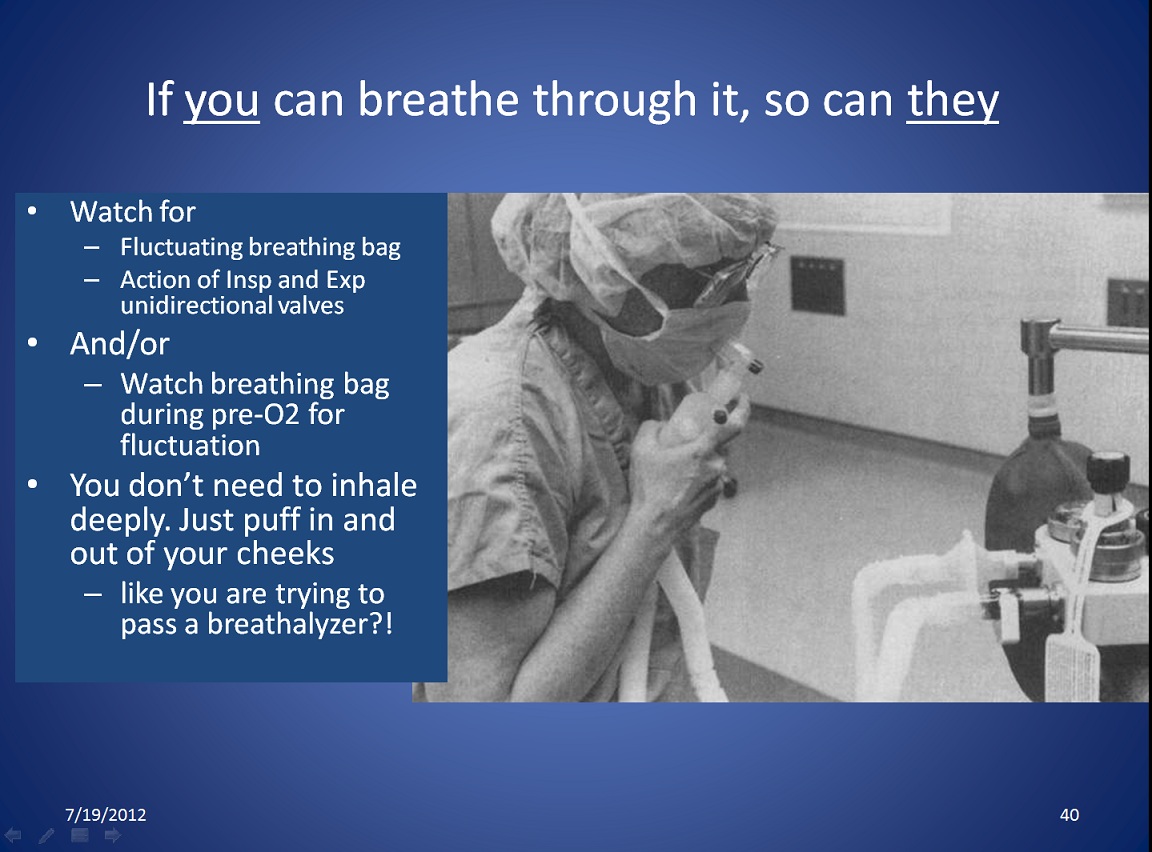
|
Breathing through it is a way to check that gas flow in the breathing circuit is not obstructed. Click on the link to see the larger version. Users may not want to breathe through the circuit for hygienic reasons, or to avoid exposure to gases or vapors. Another perhaps better way is to immediately look at the breathing bag every time you put a mask on to preoxygenate. A fluctuating breathing bag with respiration shows that there is no obstruction, and that valves are performing as expected. A third way and perhaps the best way to check for unobstructed flow through the breathing circuit was the PaF test proposed by Prien in 2019. [3] See the section below, "AWS (Anesthesia Workstation) Quick-Check & PaF test." |
Regardless of the model of gas machine, users must be able to answer at least three patient safety questions affirmatively upon completion of the electronic or automatic portion of the checklist:
Newer machines have system checkout routines that are electronic and automated. The operator follows instructions to activate flows of gases, occlude the breathing circuit during the leak check, switch from manual to mechanical ventilation, open and close the pop off valve, and manually check various functions (suction, or emergency oxygen cylinder supply). All these machine checklists require users to check certain aspects on their own, and these aspects vary from machine to machine, which creates a need for training on each machine anesthetists use.
Electronic checklists can be expected to cover most or all the steps of the PAC 2008, but this is apparent only after some study, because each checklist differs in important respects. Models may differ on whether (or how) they check oxygen monitoring, vaporizer leaks, etc. Electronic checklists may (e.g. Aisys) require that the gas analysis aspiration sampling line is disconnected before the breathing circuit is occluded by attaching it to a post. Apollo requires that the sampling line remains connected. Electronic checklists may (or may not) require the operator to repeat leak tests with each vaporizer turned on. It is not apparent what each machine checks during the autonomous portion of the morning checkout, even after consulting the manuals. Local departments must create checklist procedures for each type of workstation they own.
Electronic system checkout is logged, but may be bypassed in an emergency. Though the normal morning checklist takes only 5 minutes (more or less), the operator can perform other tasks simultaneously (such as filling syringes), so it does not appreciably slow morning preparation, unless one had not been accustomed to performing a morning gas machine checklist at all (!).
Recently a quick protocol to ensure readiness was proposed. [3] The AWS-QUICKcheck verifies four things. It is performed as each patient is brought to the OR, during pre-oxygenation, and just after induction agents. Step 2 is my addition:
Pressure and Flow (PaF) check combines leak check (step 12 above, of the PAC 2008) and flow check (step 13). It is performed before the patient is connected and takes only a few seconds.
While there is no universally accepted machine checklist less than the full PAC, situations do arise in anesthesia (e.g. for trauma or emergency cesarean section) where there is neither time nor opportunity to fully check the anesthesia gas machine. The PAC 2008 states "The PAC is essential to safe care, but should not delay initiating care if the patient needs are so urgent that time taken to complete the PAC could worsen the patient’s outcome."
Building in good habits as you start every anesthetic will help you during emergencies. This includes:
With all new machines, the electronic checklist can be bypassed in emergencies. Whether the quick minimum test above is acceptable must be determined by each clinical practice. It has been suggested that workstations be left on if trauma or obstetric cases must be done on a moment's notice. [4] The Aisys checklist can be bypassed an indefinite number of times, but it will display a visible message until the electronic checkout is performed.
This modification of the checklist was agreed upon after local peer review; it is suggested that peer review should occur in other settings where such a modification is contemplated.
|
Introduction The anesthesia gas machine must be equipped with an ascending bellows ventilator and certain monitors (capnograph, pulse oximeter, oxygen analyzer, spirometer, breathing system pressure monitor with high and low pressure alarms). If not so equipped, the checklist must be modified.
|
Risk Management encompasses pre and post-op visits, avoiding treating patients indifferently, maintaining vigilance and high standards of care, peer review, and continuing education. For anesthesia equipment, it means daily checks and appropriate maintenance. The Safe Medical Device Act 1990 mandates a report to the FDA when equipment contributes to severe injury or death.
Preventive maintenance should be done at regular intervals as called for in the operating manuals by qualified, factory-trained and approved service technicians. Vaporizers should be inspected, tested and calibrated per manufacturer's guidelines.
Quality assurance deals with objective, systematic monitoring, and the evaluation of the quality and appropriateness of patient care. Waste anesthesia gas testing can help to protect personnel and identify machines with problems. Anesthesia personnel can be held liable for knowledge of material in the anesthesia gas machine operating manual, maintenance guide, and any warnings given by the manufacturer (which are monitored and approved by the FDA the same way drug package inserts are).
Equipment that is available and functional helps anesthetists ensure patient safety.
"The chief value in adequate physical equipment is not that it makes eternal vigilance unnecessary, but that it makes eternal vigilance effective." (from 1941).Capnography and pulse oximetry are so ubiquitous, that they may be considered integral parts of the machine itself. Standards mandate a safety check daily and between cases (as needed), preventive maintenance, and machines that conform to national and state standards (e.g. some states have standards for office based anesthesia).
It is controversial whether equipment like breathing circuits can transmit infection. Most, if not all, would agree that sterilization is essential after use on a patient with known or suspected infection of the respiratory tract, especially with virulent organisms. Likewise, we should protect compromised patients from contamination arising from our equipment. In any case, handwashing between patients, as well as universal precautions, are mandatory in anesthetic practice.
See Using Anesthesia machines as ICU ventilators during Covid-19 for more.
Housekeeping during administration of anesthesia will limit the spread of contamination:
Cleaning equipment means removal of foreign matter without special attempts to kill microorganisms. Equipment should be pre-rinsed as soon as possible after use to prevent drying of organic material; then soaked, removal of soil, rinsing and drying.
Useful for heat sensitive equipment, but recontamination possible during drying and re-wrapping. Of several agents (chlorhexidine [Hibitane®], phenolic compounds, hexachlorophene, ethyl or isopropyl alcohols), glutaraldehyde is the only one effective against both tubercule bacillus and viruses, but its vapors are a health hazard.
Ethylene oxide (ETO) is a synthetic gas widely used, especially for heat or moisture-sensitive items like rubber and plastic. Kills bacteria, spores, fungi, larger viruses. Can be various patient reactions if not aerated (in wrapper) sufficiently after ETO exposure. The gas is also explosive and toxic.
Gamma radiation kills all bacteria, spores and viruses. Used for sterilization of disposable equipment - not practical for everyday needs of hospitals.
The Centers for Disease Control- NIOSH has a collection of useful information relating to bloodborne diseases and universal precautions.
AANA publishes Infection Control Guidelines (2025).
[1] APSF Newsletter Spring 2008. New Guidelines Available for Pre-Anesthesia Checkout at https://www.apsf.org/article/new-guidelines-available-for-pre-anesthesia-checkout/ Accessed Feb 2, 2026
[2] Mehta SP, Eisenkraft JB, Posner KL, Domino KB. Patient injuries from anesthesia gas delivery equipment: a closed claims update. Anesthesiology. 2013 Oct;119(4):788-95. doi: 10.1097/ALN.0b013e3182a10b5e.
[3] Prien T, Brinker A, Pfeiffer K, Van Aken HK. Equipment Problems. Preventable Anesthetic Deaths: Is "PaF" the Magic Dragon? Anesth Analg. 2019 Nov;129(5):1439-1441. doi: 10.1213/ANE.0000000000004408.
[4] Gross JB. Draeger Narkomed 6000 poses patient safety risks. Anesthesiology. 2001 Aug;95(2):567-8. doi: 10.1097/00000542-200108000-00052.